
W = QV, so an electron, having a charge 'e' will obtain energy of XeV if placed in an potential difference of X volts.
ATOMIC SPECTRA LINE SPECTRA FREE
It will take just the amount of energy it needs for the jump from the free electron's kinetic energy.Īn electron can obtain energy from an electrical supply. Obtaining energy by colliding with a free electron. Photon absorption - absorbing a photon of exactly the correct energy needed for a 'jump'. It can obtain the energy required for 'promotion' to a higher energy state by several means: Of an atom it either absorbs energy for the transition or emits a photon when reducing its potential energy.

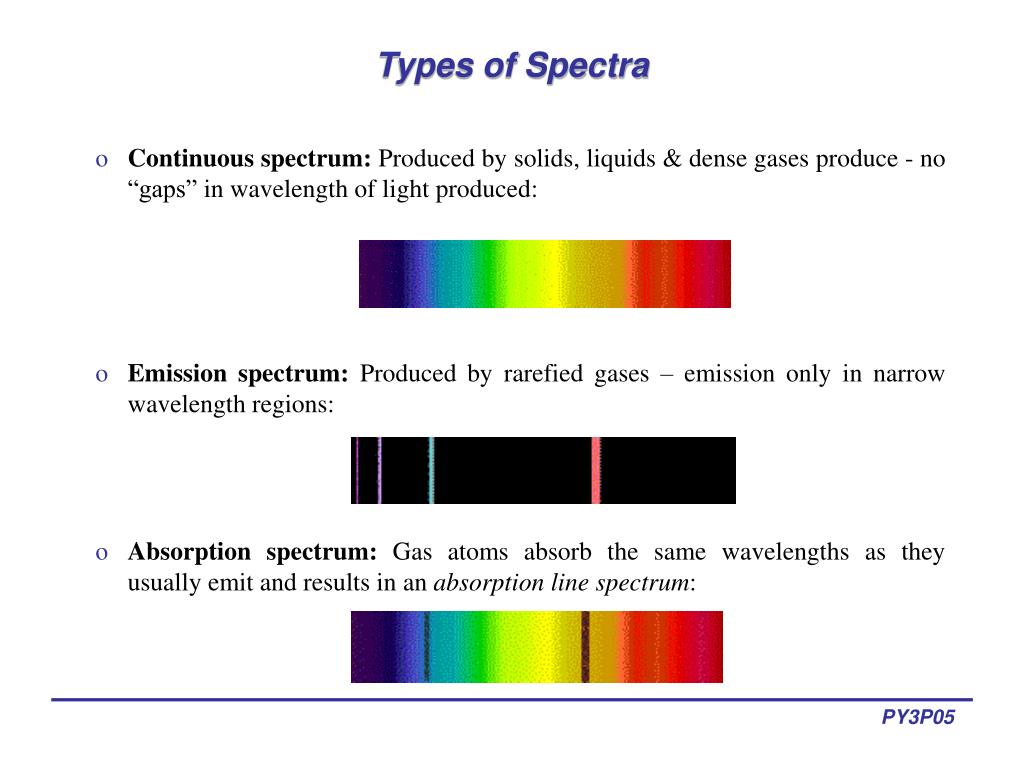
When an electron jumps between energy levels We can put information about these energy states into a diagram When it is closer to the nucleus it has less potential energy (needsĮnergy input to escape the influence). Nucleus' influence it is said to have zero potential energy - a zero energy The further away from the nucleus these states are, the higher the potentialĮnergy of the electron in that state. Now increase the speed of the simulation in order to fill in the signal collected by the spectrometer and observe the results.Energy states within the atom (called orbitals by chemists).Observe what happens when the electron relaxes back down to a lower energy level. Observe what happens with the photons when the electron jumps to a higher energy level. Recall that a neutral hydrogen only has one electron, which is represented by the blue dot rotating around the energy levels of the atom in this simulation.Also check the box to show the electron energy level diagram. Each photon corresponds to that color dot with fuzzy purple dots representing UV. Turn on the gun to shoot photons (“packets of light” or “energy bundles”) by clicking the red button. Click the box to set the Light Control to “White”. Using a computer or a mobile device, access the " Models of the Hydrogen Atom" simulation from . Set the dial to “Prediction” and select Bohr's Atomic Model.In fact, flame tests were used to identify elements long before the invention of modern techniques, such as emission spectroscopy. As many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. This one color results from a combination of all lines of the emission spectrum, in proportion to their intensities. For example, helium gas when excited by an electrical discharge emits light that appears an orange- peach color. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. Unfortunately, techniques more sophisticated than those used in this lab are required to obtain such line spectra. For example, the line spectra shown below for the elements helium and carbon are clearly quite different. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. If emitted photons are in the visible region of the spectrum, they may be perceived as lines of different colors (note that photons outside the visible spectrum may also be emitted, but cannot be seen). The spacing between energy levels in an atom determines the sizes of the transitions that occur, and thus the energy and wavelengths of the collection of photons emitted. However, when electrons subsequently return from higher energy levels to lower energy levels, energy is released predominantly in the form of electromagnetic radiation. The energy absorbed could be in the form of heat (as in flame tests), or electrical energy, or electromagnetic radiation. So, how does electromagnetic radiation relate to flame tests? Well, when an atom or ion absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels.
Other examples of electromagnetic radiation include X-rays, ultraviolet light, infrared light, microwaves and radio waves.
ATOMIC SPECTRA LINE SPECTRA PDF
Differences in the wavelengths of visible light are manifested as different colors, shown in the color spectrum below (colors can be seen in the PDF document on-line). Visible light is the most familiar example of electromagnetic radiation.


 0 kommentar(er)
0 kommentar(er)
